Neuroscientists still have a tremendous amount to learn about the causes and courses of neurodegenerative diseases and Down syndrome, but as speakers at the Oct. 5-6 MIT symposium “Glial and Neuronal Biology of the Aging Brain” pointed out, often when they make a new discovery in the context of one such condition, it teaches them something relevant to others.
“Our belief is that the study of the aging brain can learn a great deal from the study of Down syndrome and vice versa,” said Picower Professor Li-Huei Tsai who directs the two MIT entities that jointly hosted the conference: The Aging Brain Initiative and the Alana Down Syndrome Center. “It would be a wonderful outcome of this symposium if we can play even a small role in bringing these two communities of scientists, physicians, and engineers, and even caregivers closer together.”
The event indeed marshaled a multitude of online attendees. Over the course of the two-day program more than 400 people tuned in from 27 countries. They heard scientists from places as far-ranging as Hong Kong and Germany share their latest research and discuss the many intersections they see among Alzheimer’s and other dementias, Parkinson’s disease, Huntington’s disease and Down syndrome.
For example, Tracy Young Pearse, associate professor of neurology at Harvard Medical School and Brigham and Women’s Hospital, discussed her lab’s new finding that the three copies of the genes APP and DYRK1A found in Down syndrome neurons (because they have three copies of chromosome 21), increase phosphorylated tau (a pathological hallmark of Alzheimer’s) and promote excessive transport and release of neurotransmitters across connections with other neurons, a potential source of circuit dysfunction.
Vessels of concern
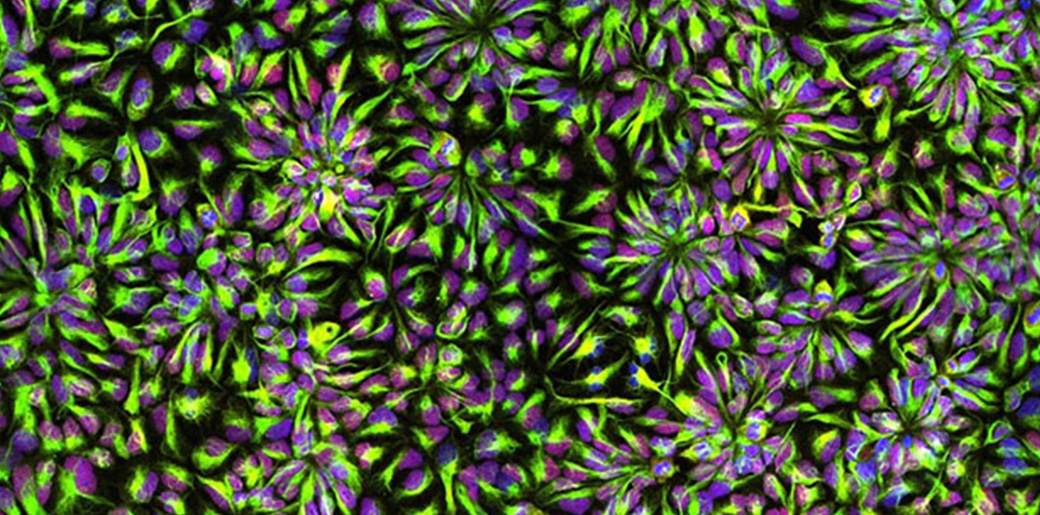
Though neural circuits remain at the heart of brain function, three speakers instead focused their talks on the brain’s circulatory system. MIT Associate Professor Myriam Heiman noted that the breakdown of the blood-brain barrier, which strictly filters what the body and brain exchange, are suspected of being key contributor to many neurodegenerative diseases. In presenting her lab’s new research that produced a novel “atlas” of cell types in the brain’s blood vessels, she showed clear evidence that vascular integrity is weakened in Huntington’s disease and that the degradation is associated with a problematic innate immune response.
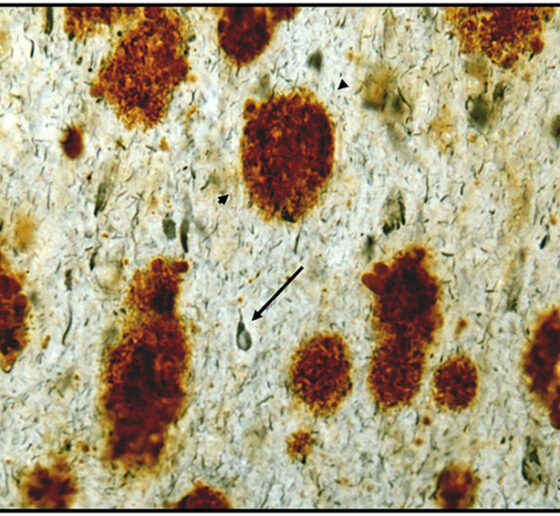
Elizabeth Head, Professor of pathology and laboratory medicine at the University of California at Irvine, related dysfunction of brain vasculature to the connection between Down syndrome and Alzheimer’s. Though people with Down syndrome are relatively protected against cardiovascular problems such as high blood pressure or atheroma, an excess of amyloid protein in their brain blood vessels leads to cerebral amyloid angiopathy, a condition closely associated with Alzheimer’s. Head’s lab has shown that people with Down syndrome and CAA exhibit microbleeds along their brain blood vessels.
Head collaborates with Adam Brickman, professor of neuropsychology at Columbia University. He presented recent studies showing that magnetic resonance imaging of “white matter hyperintensities” and other vascular problems can be a biomarker of Alzheimer’s pathology in people with Down syndrome. The hyperintensities, which the team showed to be especially prevalent in posterior lobes of the brain, are believed to be the result of brain vasculature problems and correlated with other problems such as microbleeds.
Cells not immune from scrutiny
Several other speakers focused on the brain’s immune cells, called microglia, which have a very complex role in neurodegenerative diseases including Alzheimer’s.
Microglia, for instance, take on many different states in Alzheimer’s ranging from beneficial to harmful. In her talk, Harvard Medical School & Boston Children’s Hospital neurology Associate Professor Beth Stevens described methods her lab has developed for culturing microglia from stem cells and then coaxing them into these many states by tailoring either their genetic background, their environmental context, or both.
Li Gan, professor of neuroscience at Weill Cornell Medicine, discussed particular instances in which molecularly manipulating microglial state can sustain the brain’s resilience to Alzheimer’s pathology. In a study published earlier this year her lab found that by reducing expression of the gene transcription factor NFkappaB in microglia, the lab could reduce spreading of the problematic protein tau. She also shared even newer results showing that intervening in a specific runaway immune pathway in microglia by knocking down a key molecule, her lab has shown benefits in learning and memory in mice. The method appears to do so by increasing activity of a resilience-promoting transcription factor called MEF2 that Tsai’s lab has also independently identified as beneficial.
Hong Kong University of Science and Technology Professor Nancy Yuk-Yu Ip detailed another molecular method of helping microglia combat Alzheimer’s. Her lab has found that in the disease a soluble form of the molecule ST2 intercepts the immune molecule interleukin 33 (IL-33), which would normally prompt a transition of microglia into a beneficial state. The lab has shown that injecting IL-33 improves Alzheimer’s pathology in mice and has found a genetic variant in people that conveys protection against this problem.
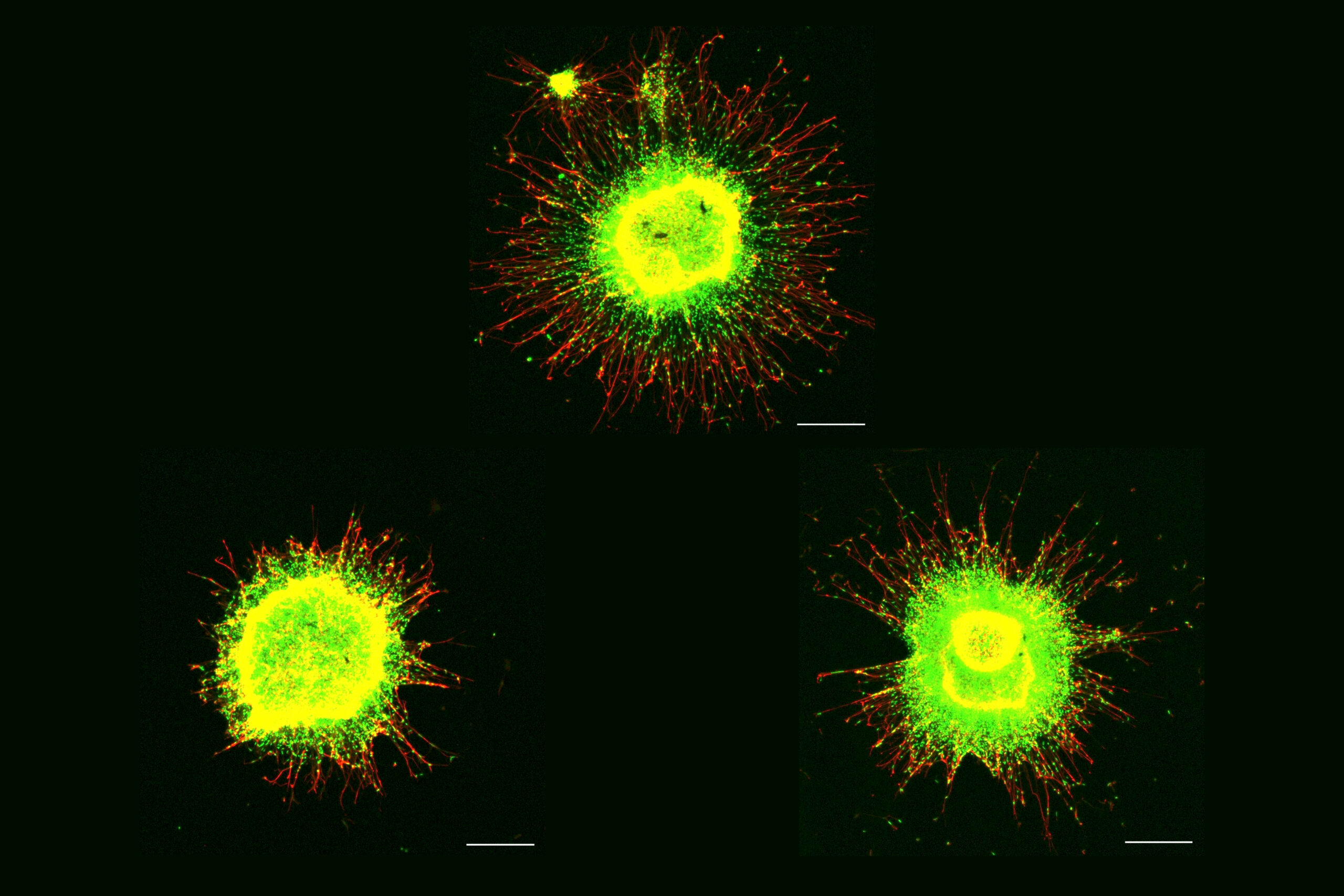
In his talk, Michael Heneka, director of the Luxembourg Centre for Systems Biomedicine, showed how microglia literally throw neurons a line to help them fight back against toxic proteins. His lab found that microglia extend “tunneling nanotubes” to neurons beset with tau (a toxic aggregate in some dementias) or alpha-synuclein (a toxic aggregate most prevalent in Parkinson’s disease) to remove the proteins and to supply neurons with fresh mitochondria to rescue them from oxidative stress.
A system with many parts
Neurons, vascular cells, and microglia were not the only cells with time in the spotlight. Shane Liddelow, assistant professor of neuroscience and physiology at New York University focused on astrocytes, an abundant cell type in the brain with key roles in supporting neural function and linking neurons to blood vessels. He shared new research indicating that subtypes of astrocytes have inflammatory responses in disease and in the case of Alzheimer’s, associate with pathology in particular parts of the brain. Further research can help determine what those subtypes may matter to the progression of the disease.
Astrocytes, neurons and microglia were all featured in the remarks of Gilbert Di Paolo, executive director of discovery biology at Denali Therapeutics. He discussed the company’s potential therapy for a subset of cases of frontotemporal dementia. In those cases, mutations reduce levels of progranulin, which undermines the function of cells’ lysosomes. By restoring levels of progranulin in cells the company is restoring lysosomal function and therefore indicators of cell health.
Complementing the talks’ exposition of the variety of cell types and molecular mechanisms at issue across neurodegenerative diseases and Down syndrome were the posters of MIT postdocs and graduate students that followed the talks. A dozen presenters from seven labs affiliated with the Aging Brain Initiative, the Alana Center, or both highlighted whole systems approaches to understanding and treating disease. Members of Tsai’s lab, for instance, discussed the therapeutic possibilities for Down syndrome of stimulating the brain with light and sound at the key frequency of 40Hz. Members of the labs of Professors Ed Boyden and Alan Jasanoff presented new advances in brain imaging. Members of Professor Manolis Kellis’s lab showed how sophisticated computational approaches can help demystify the genetic complexities of Down syndrome. A poster representing the lab of Professor Ernest Fraenkel highlighted molecular networks related to neurodegeneration. And members of the labs of Professors Ann Graybiel and Matthew Wilson highlighted neural mechanisms fundamental to behavior and memory.
The symposium offered all these scientists, and their hundreds of audience members, the chance to virtually gather and learn from each other at a crossroads of intersecting disease biology.