Category: News & events
Symposium examines intersecting biology of neurodegeneration, Down syndrome
Talk Videos
Watch videos of several of the symposium talks on YouTube.
Neuroscientists still have a tremendous amount to learn about the causes and courses of neurodegenerative diseases and Down syndrome, but as speakers at the Oct. 5-6 MIT symposium “Glial and Neuronal Biology of the Aging Brain” pointed out, often when they make a new discovery in the context of one such condition, it teaches them something relevant to others.
“Our belief is that the study of the aging brain can learn a great deal from the study of Down syndrome and vice versa,” said Picower Professor Li-Huei Tsai who directs the two MIT entities that jointly hosted the conference: The Aging Brain Initiative and the Alana Down Syndrome Center. “It would be a wonderful outcome of this symposium if we can play even a small role in bringing these two communities of scientists, physicians, and engineers, and even caregivers closer together.”
The event indeed marshaled a multitude of online attendees. Over the course of the two-day program more than 400 people tuned in from 27 countries. They heard scientists from places as far-ranging as Hong Kong and Germany share their latest research and discuss the many intersections they see among Alzheimer’s and other dementias, Parkinson’s disease, Huntington’s disease and Down syndrome.
For example, Tracy Young Pearse, associate professor of neurology at Harvard Medical School and Brigham and Women’s Hospital, discussed her lab’s new finding that the three copies of the genes APP and DYRK1A found in Down syndrome neurons (because they have three copies of chromosome 21), increase phosphorylated tau (a pathological hallmark of Alzheimer’s) and promote excessive transport and release of neurotransmitters across connections with other neurons, a potential source of circuit dysfunction.
Vessels of concern
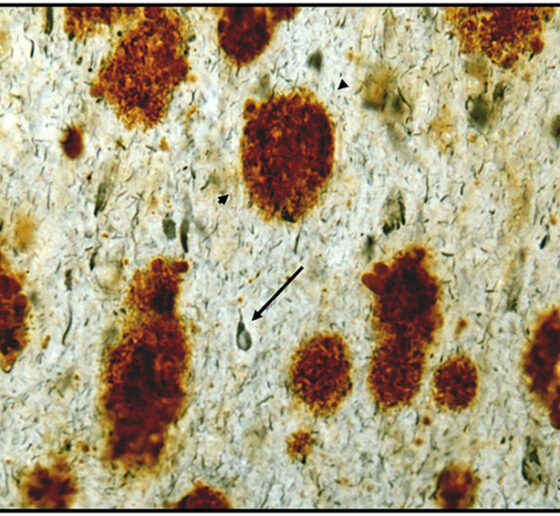
Though neural circuits remain at the heart of brain function, three speakers instead focused their talks on the brain’s circulatory system. MIT Associate Professor Myriam Heiman noted that the breakdown of the blood-brain barrier, which strictly filters what the body and brain exchange, are suspected of being key contributor to many neurodegenerative diseases. In presenting her lab’s new research that produced a novel “atlas” of cell types in the brain’s blood vessels, she showed clear evidence that vascular integrity is weakened in Huntington’s disease and that the degradation is associated with a problematic innate immune response.
Elizabeth Head, Professor of pathology and laboratory medicine at the University of California at Irvine, related dysfunction of brain vasculature to the connection between Down syndrome and Alzheimer’s. Though people with Down syndrome are relatively protected against cardiovascular problems such as high blood pressure or atheroma, an excess of amyloid protein in their brain blood vessels leads to cerebral amyloid angiopathy, a condition closely associated with Alzheimer’s. Head’s lab has shown that people with Down syndrome and CAA exhibit microbleeds along their brain blood vessels.
Head collaborates with Adam Brickman, professor of neuropsychology at Columbia University. He presented recent studies showing that magnetic resonance imaging of “white matter hyperintensities” and other vascular problems can be a biomarker of Alzheimer’s pathology in people with Down syndrome. The hyperintensities, which the team showed to be especially prevalent in posterior lobes of the brain, are believed to be the result of brain vasculature problems and correlated with other problems such as microbleeds.
Cells not immune from scrutiny
Several other speakers focused on the brain’s immune cells, called microglia, which have a very complex role in neurodegenerative diseases including Alzheimer’s.
Microglia, for instance, take on many different states in Alzheimer’s ranging from beneficial to harmful. In her talk, Harvard Medical School & Boston Children’s Hospital neurology Associate Professor Beth Stevens described methods her lab has developed for culturing microglia from stem cells and then coaxing them into these many states by tailoring either their genetic background, their environmental context, or both.
Li Gan, professor of neuroscience at Weill Cornell Medicine, discussed particular instances in which molecularly manipulating microglial state can sustain the brain’s resilience to Alzheimer’s pathology. In a study published earlier this year her lab found that by reducing expression of the gene transcription factor NFkappaB in microglia, the lab could reduce spreading of the problematic protein tau. She also shared even newer results showing that intervening in a specific runaway immune pathway in microglia by knocking down a key molecule, her lab has shown benefits in learning and memory in mice. The method appears to do so by increasing activity of a resilience-promoting transcription factor called MEF2 that Tsai’s lab has also independently identified as beneficial.
Hong Kong University of Science and Technology Professor Nancy Yuk-Yu Ip detailed another molecular method of helping microglia combat Alzheimer’s. Her lab has found that in the disease a soluble form of the molecule ST2 intercepts the immune molecule interleukin 33 (IL-33), which would normally prompt a transition of microglia into a beneficial state. The lab has shown that injecting IL-33 improves Alzheimer’s pathology in mice and has found a genetic variant in people that conveys protection against this problem.
In his talk, Michael Heneka, director of the Luxembourg Centre for Systems Biomedicine, showed how microglia literally throw neurons a line to help them fight back against toxic proteins. His lab found that microglia extend “tunneling nanotubes” to neurons beset with tau (a toxic aggregate in some dementias) or alpha-synuclein (a toxic aggregate most prevalent in Parkinson’s disease) to remove the proteins and to supply neurons with fresh mitochondria to rescue them from oxidative stress.
A system with many parts
Neurons, vascular cells, and microglia were not the only cells with time in the spotlight. Shane Liddelow, assistant professor of neuroscience and physiology at New York University focused on astrocytes, an abundant cell type in the brain with key roles in supporting neural function and linking neurons to blood vessels. He shared new research indicating that subtypes of astrocytes have inflammatory responses in disease and in the case of Alzheimer’s, associate with pathology in particular parts of the brain. Further research can help determine what those subtypes may matter to the progression of the disease.
Astrocytes, neurons and microglia were all featured in the remarks of Gilbert Di Paolo, executive director of discovery biology at Denali Therapeutics. He discussed the company’s potential therapy for a subset of cases of frontotemporal dementia. In those cases, mutations reduce levels of progranulin, which undermines the function of cells’ lysosomes. By restoring levels of progranulin in cells the company is restoring lysosomal function and therefore indicators of cell health.
Complementing the talks’ exposition of the variety of cell types and molecular mechanisms at issue across neurodegenerative diseases and Down syndrome were the posters of MIT postdocs and graduate students that followed the talks. A dozen presenters from seven labs affiliated with the Aging Brain Initiative, the Alana Center, or both highlighted whole systems approaches to understanding and treating disease. Members of Tsai’s lab, for instance, discussed the therapeutic possibilities for Down syndrome of stimulating the brain with light and sound at the key frequency of 40Hz. Members of the labs of Professors Ed Boyden and Alan Jasanoff presented new advances in brain imaging. Members of Professor Manolis Kellis’s lab showed how sophisticated computational approaches can help demystify the genetic complexities of Down syndrome. A poster representing the lab of Professor Ernest Fraenkel highlighted molecular networks related to neurodegeneration. And members of the labs of Professors Ann Graybiel and Matthew Wilson highlighted neural mechanisms fundamental to behavior and memory.
The symposium offered all these scientists, and their hundreds of audience members, the chance to virtually gather and learn from each other at a crossroads of intersecting disease biology.
Upcoming Alana Down Syndrome/Aging Brain Initiative Symposium
The topic of this symposium is Glial and Neuronal Biology of the Aging Brain
REGISTER HERE:
-
Adam M. Brickman, Columbia University
-
Gilbert Di Paolo, Denali Therapeutics
-
Li Gan, Weill Cornell Medical College
-
Elizabeth Head, University of California, Irvine
-
Myriam Heiman, Picower Institute, MIT
-
Michael Heneka, Luxembourg Centre for Systems Biomedicine (LCSB)
-
Nancy Yuk-Yu Ip, The Hong Kong University of Science and Technology
-
Shane Liddelow, NYU
-
Beth Stevens, Boston Children’s Hospital, Harvard Medical School
-
Tracy Young-Pearse, Brigham and Women’s Hospital, Harvard Medical School
Watch: Technology to Improve Ability Webinar

The ADSC hosted an exciting webinar showcasing two projects funded by the Innovation to Improve Ability program at the Deshpande Center for Technical Innovation at MIT.
Watch the full Webinar below!
Upcoming Webinar: Technology to Improve Ability

The ADSC presents an update on our Technology to Improve Ability Grant program. These talks will be accessible to the general public.
Featured Talks:
A Novel Device For Obstructive Sleep Apnea In Down Syndrome
(Ravi Rasalingam and Tarsha Ward of the Ellen Roche group)
&
Commalla: Augmentative Communication Tech using Naturalistic Data and Machine Learning
(Kristina Johnson and Jaya Narain of the Pattie Maes and Rosalind Picard groups)
Registration is free, but required: https://mit.zoom.us/webinar/register/WN_MhEkEpxzQnyXqJ55OFnx0g
Down syndrome symposium highlights clinical, fundamental progress

Whether they are working with patients in clinical trials or with chromosomes in cell cultures, scientists and physicians in the Boston area and beyond are testing a wide variety of new ways help people with Down syndrome. At the New England Down Syndrome Symposium, presented by the Alana Down Syndrome Center on Nov. 10, a virtual audience of hundreds of people learned about the research progress of a dozen research teams. The Alana Center at MIT partnered with the Massachusetts Down Syndrome Congress and the LuMind IDSC Foundation to organize the daylong program of online talks.
“I am hopeful that the research being done today will improve medical care and the quality of life of people with Down syndrome,” said Kate Bartlett, a member of the Self-Advocate Advisory Council of the MDSC. “Your work is important for me and my peers. Together we can make a better world for all people to lead active, healthy, fulfilling lives.”
Clinical studies
One of the specific health concerns Bartlett, who is 35, called out in her remarks is that the age of onset for Alzheimer’s disease among people with Down syndrome can be as early as 40. Finding ways to address the community’s elevated risk of Alzheimer’s was one of the four main themes of the symposium, along with new potential therapies for sleep apnea, and fundamental research on developmental biology and on chromosome number and dosage.
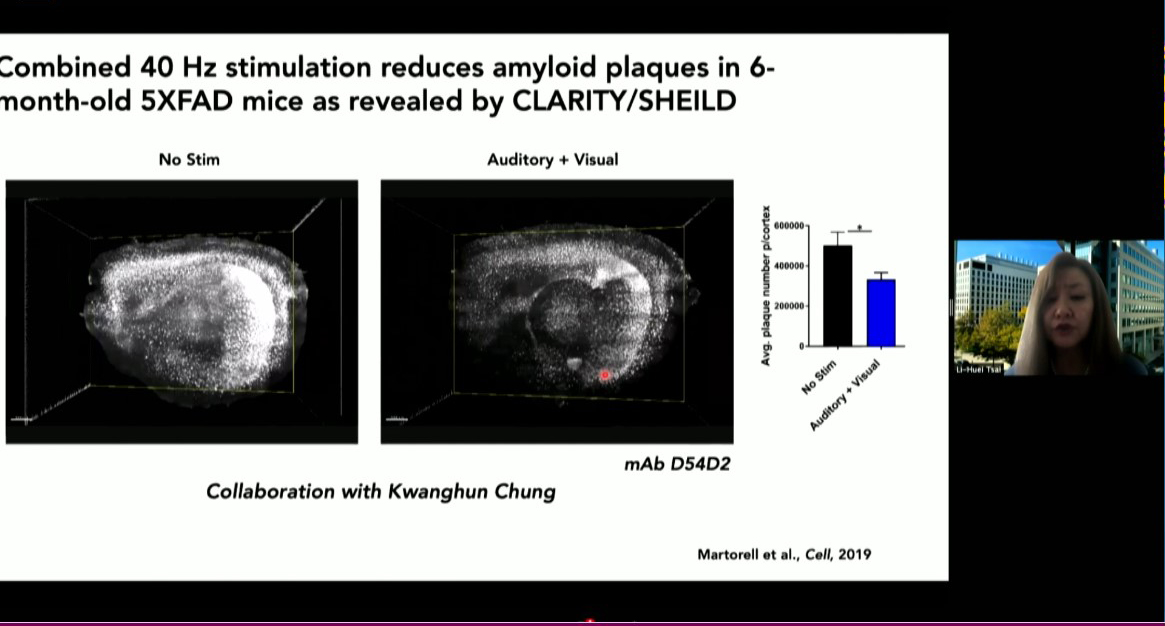
MIT is poised to launch a clinical study of a potential Alzheimer’s therapy among people with Down syndrome, said Alana center co-director Li-Huei Tsai, Picower Professor of Neuroscience at MIT. About five years ago her lab discovered that in Alzheimer’s brain wave power and connectivity at a specific frequency, 40Hz, is notably lessened. They discovered that by exposing lab mice to light flickering and sound buzzing at 40Hz they could restore the rhythm, leading to many benefits including improved learning and memory, reduced neuron death and reductions in the level of toxic tau and amyloid proteins considered hallmarks of Alzheimer’s pathology.
More recently the team has begun clinical studies of the potential therapy, called Gamma ENtrainment Using Sensory Stimuli (GENUS), in humans to test its safety and efficacy in healthy people and in people with Alzheimer’s. Picower Clinical Fellow Diane Chan, the neurologist leading the human studies, said that so far the data indicate that exposure to 40Hz light and sound is safe and may be contributing to improved sleep and a preservation of brain volume in patients with mild Alzheimer’s disease. As soon as conditions related to the Covid-19 pandemic allow, she said, the team will invite people with Down syndrome to enroll in a study to test safety, tolerability and efficacy specifically for them.
Two speakers from Massachusetts General Hospital tackled the important related issue of diagnosing and tracking the progression of Alzheimer’s specifically in people with Down syndrome. Stephanie Santoro, a clinical geneticist with the hospital’s Down syndrome program, described the nationwide LIFE-DSR study, which counts Bartlett among its participants. The study has rigorously developed a suite of assessments to track changes in cognition, behavior, function and health in 270 adults of various ages with Down syndrome over a course of more than 30 months, Santoro said. The results will offer doctors and patients new insights into how aging and Alzheimer’s affect life over time, which may help in screening for Alzheimer’s risk.
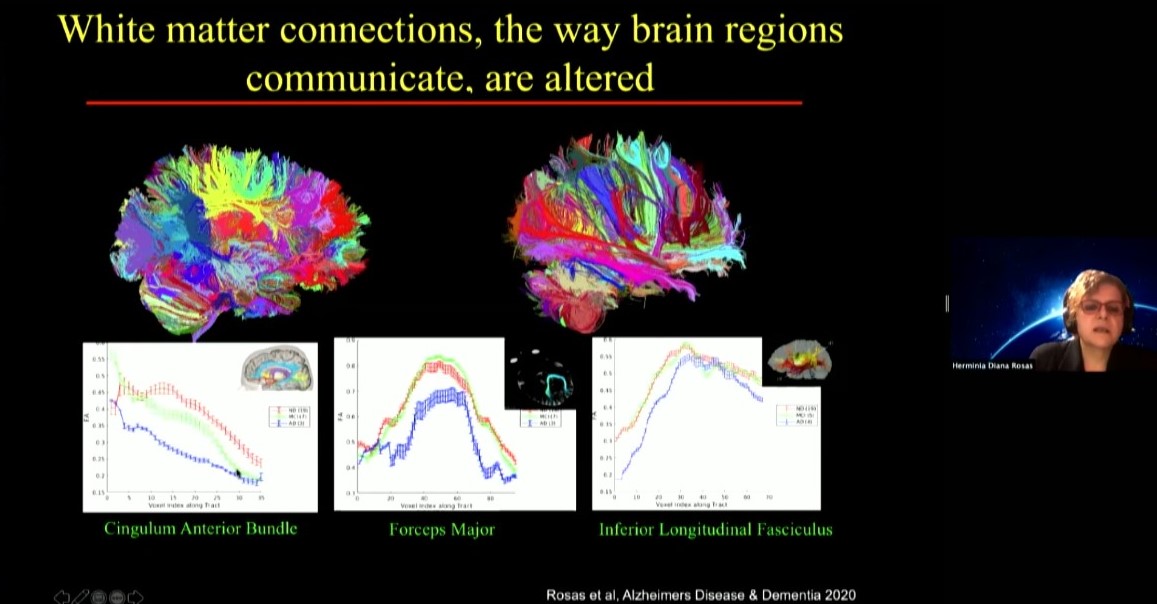
Diana Rosas, an MGH neurologist, is one of the researchers helping to run the LIFE-DSR study. In her talk she focused on another study, the National Institute of Health’s ABC-DS study, in which she is developing biomarkers that may indicate the onset of mild cognitive impairment and Alzheimer’s in Down syndrome including data from brain scans, molecule and protein levels measured in blood, and genetic screens. She noted that these markers seeking to track changes over time need to be specific for Down syndrome patients, for instance because they have characteristic differences in brain anatomy compared to people who don’t have the condition.
Another challenge that can hinder learning, memory and cognition in people with Down syndrome is loss of sleep due to breathing trouble. Two symposium speakers discussed new approaches to treating the problem, called sleep apnea, which is very common in people with Down syndrome because of characteristics such as decreased muscle tone, differences in facial anatomy, and larger tongue size. Daniel Combs, a pediatrician at the University of Arizona, described a trial he recently began to test a combination of drugs to treat sleep apnea in children with Down syndrome. The medicines he’s testing have been studied for sleep apnea in non-Down syndrome adults and appear to be helping by increasing airway muscle tone, he said.
Massachusetts Eye and Ear Infirmary otolayrngologist Christopher Hartnick, meanwhile, discussed a surgical approach he is testing for difficult sleep apnea cases. Called hypoglossal nerve stimulation, the procedure involves implanting a breathing sensor on a rib that leads to a processor further up the chest. The processor then stimulates electrodes on muscles of the tongue. When the patient is drawing a breath (sensed at the rib) the processor stimulates the tongue muscles to move the tongue out of the way to improve air flow. This approach has been successful in adults with DS and sleep apnea. So far, Hartnick said, 33 children have been implanted and results look promising.
Fundamental research
At the same time that all of these clinical trials have been progressing, other researchers have been working in the lab to advance more fundamental understanding of the biology going on in cells of people with Down syndrome, often also called trisomy 21 because it is caused by having a third copy of chromosome 21.
Some researchers have forged ahead by working to develop better mouse models of Down syndrome that can more closely reproduce the biology of the condition in the lab. Tarik Haydar of the Center for Neuroscience Research at Children’s National Hospital in Washington DC described his lab’s recent study showing how variations in a predominant mouse model called Ts65dn have led to differing and sometimes contradictory research conclusions that need to be recognized and accounted for.
While important nuances about the Ts65dn model are becoming better understood, Elizabeth Fisher of University College London shared that new mouse models are emerging. In mice the genes that are on human chromosome 21 are spread out over three chromosomes. That has given researchers the challenge of engineering mice to express genes in the same way that people with Down syndrome do. Fisher’s lab has led advances in doing so, and she reported that recently another group managed to directly inserting human chromosome 21 into mice to develop a new model, the TcMAC21 mouse.
Mouse models are crucial because they are whole living organisms that can demonstrate how health and behavior change with an extra chromosome. But another way to model Down syndrome in the lab is by engineering human cell cultures from cells taken from patients. Skin cells, for instance, can be turned into stem cells, which in turn can grow into neurons or heart cells.
MIT biology Professor Laurie Boyer, for instance, has begun studying gene expression in heart muscle cells derived from Down syndrome (DS) persons. The development of the heart is a very intricate and sensitive process and faulty regulation leads to congenital heart defects (CHD). Her goal is to learn how an extra copy of chromosome 21 in DS contributes to the high incidence of CHD that will hopefully fuel potential new therapies for these heart defects.
In other experiments with patient-derived cells—in this case, neurons—Lindy Barrett of the Broad Institute of MIT and Harvard is finding intriguing overlaps between Down syndrome and a form of autism called Fragile X syndrome. Her lab is finding that the protein missing in Fragile X, called FMRP, normally regulates some genes that are also expressed too much in Down syndrome. The findings, she said, raise the question of whether manipulating levels of FMRP could help Down syndrome patients.
While individual genes, or groups of them, could present new targets for therapies, another goal of the field remains finding a way to repress the activity of the third chromosome 21 as a whole. Speakers Jeanine Lee and Mitzi Kuroda, each of Harvard Medical School, described mechanisms by which various organisms, including humans, naturally suppress or enhance whole-chromosome activity. Females have two X chromosomes but males have an X and a Y. To remedy that imbalance, insects like fruit flies doubly express the X chromosome in males but mammals, like people, suppress or “silence” the activity of one of the X chromosomes in females.
This unusual degree of whole-chromosome up- or down-regulation offers intriguing scientific opportunities. Lee discussed how she hopes to address an autism-like disorder called Rett Syndrome in which girls develop abnormally because a mutant copy of the gene MeCP2 happens to be on one X chromosome they express. Her strategy is to selectively subvert X-chromosome silencing to express the healthy copy of MeCP2 on the inactivated X chromosome. Meanwhile speaker Stefan Pinter of the University of Connecticut discussed how his lab is using the X-chromosome’s silencing machinery to silence the extra chromosome 21 in Down syndrome. Pinter said that by silencing the third copy in developing brain cells in the lab his research group is developing a dynamic model for lab studies in which they can now control chromosome 21 dosage in developing brain cells.
In wrapping up the symposium, Alana Center faculty member Ed Boyden, Y. Eva Tan Professor of Neurotechnology at MIT, said the day provided many individual examples of progress that taken together are even more encouraging.
“Today we have seen many individual examples of research and advocacy from which we can draw inspiration and hope,” he said. “But there is another source of those same feelings as well: the way this community came together today to share and to learn from each other. Even if our interaction was virtual, the growth in our understanding and our interconnection was real.”
Angelika Amon, research pioneer and ADSC founding member, dies at 53

MIT is mourning the passing of biologist Angelika Amon, co-director of the Alana Down Syndrome Center. She died of cancer after living with the disease for two-and-a-half years. “Angelika’s intellect and research were as astonishing as her bravery and her spirit,” said her Alana colleague Li-Huei Tsai.
Angelika Amon, professor of biology and a member of the Koch Institute for Integrative Cancer Research, died on Oct. 29 at age 53, following a two-and-a-half-year battle with ovarian cancer.
“Known for her piercing scientific insight and infectious enthusiasm for the deepest questions of science, Professor Amon built an extraordinary career – and in the process, a devoted community of colleagues, students and friends,” MIT President L. Rafael Reif wrote in a letter to the MIT community.
“Angelika was a force of nature and a highly valued member of our community,” reflects Tyler Jacks, the David H. Koch Professor of Biology at MIT and director of the Koch Institute. “Her intellect and wit were equally sharp, and she brought unmatched passion to everything she did. Through her groundbreaking research, her mentorship of so many, her teaching, and a host of other contributions, Angelika has made an incredible impact on the world — one that will last long into the future.”
A pioneer in cell biology
From the earliest stages of her career, Amon made profound contributions to our understanding of the fundamental biology of the cell, deciphering the regulatory networks that govern cell division and proliferation in yeast, mice, and mammalian organoids, and shedding light on the causes of chromosome mis-segregation and its consequences for human diseases.
Human cells have 23 pairs of chromosomes, but as they divide they can make errors that lead to too many or too few chromosomes, resulting in aneuploidy. Amon’s meticulous and rigorous experiments, first in yeast and then in mammalian cells, helped to uncover the biological consequences of having too many chromosomes. Her studies determined that extra chromosomes significantly impact the composition of the cell, causing stress in important processes such as protein folding and metabolism, and leading to additional mistakes that could drive cancer. Although stress resulting from aneuploidy affects cells’ ability to survive and proliferate, cancer cells — which are nearly universally aneuploid — can grow uncontrollably. Amon showed that aneuploidy disrupts cells’ usual error-repair systems, allowing genetic mutations to quickly accumulate.
Aneuploidy is usually fatal, but in some instances extra copies of specific chromosomes can lead to conditions such as Down syndrome and developmental disorders including those known as Patau and Edwards syndromes. This led Amon to work to understand how these negative effects result in some of the health problems associated specifically with Down syndrome, such as acute lymphoblastic leukemia. Her expertise in this area led her to be named co-director of the recently established Alana Down Syndrome Center at MIT.
“Angelika’s intellect and research were as astonishing as her bravery and her spirit. Her lab’s fundamental work on aneuploidy was integral to our establishment of the center,” say Li-Huei Tsai, the Picower Professor of Neuroscience and co-director of the Alana Down Syndrome Center. “Her exploration of the myriad consequences of aneuploidy for human health was vitally important and will continue to guide scientific and medical research.”
Another major focus of research in the Amon lab has been on the relationship between how cells grow, divide, and age. Among other insights, this work has revealed that once cells reach a certain large size, they lose the ability to proliferate and are unable to reenter the cell cycle. Further, this growth contributes to senescence, an irreversible cell cycle arrest, and tissue aging. In related work, Amon has investigated the relationships between stem cell size, stem cell function, and tissue age. Her lab’s studies have found that in hematopoetic stem cells, small size is important to cells’ ability to function and proliferate — in fact, she posted recent findings on bioRxiv earlier this week — and have been examining the same questions in epithelial cells as well.
Amon lab experiments delved deep into the mechanics of the biology, trying to understand the mechanisms behind their observations. To support this work, she established research collaborations to leverage approaches and technologies developed by her colleagues at the Koch Institute, including sophisticated intestinal organoid and mouse models developed by the Yilmaz Laboratory, and a microfluidic device developed by the Manalis Laboratory for measuring physical characteristics of single cells.
The thrill of discovery
Born in 1967, Amon grew up in Vienna, Austria, in a family of six. Playing outside all day with her three younger siblings, she developed an early love of biology and animals. She could not remember a time when she was not interested in biology, initially wanting to become a zoologist. But in high school, she saw an old black-and-white film from the 1950s about chromosome segregation, and found the moment that the sister chromatids split apart breathtaking. She knew then that she wanted to study the inner workings of the cell and decided to focus on genetics at the University of Vienna in Austria.
After receiving her BS, Amon continued her doctoral work there under Professor Kim Nasmyth at the Research Institute of Molecular Pathology, earning her PhD in 1993. From the outset, she made important contributions to the field of cell cycle dynamics. Her work on yeast genetics in the Nasmyth laboratory led to major discoveries about how one stage of the cell cycle sets up for the next, revealing that cyclins, proteins that accumulate within cells as they enter mitosis, must be broken down before cells pass from mitosis to G1, a period of cell growth.
Towards the end of her doctorate, Amon became interested in fruitfly genetics and read the work of Ruth Lehmann, then a faculty member at MIT and a member of the Whitehead Institute. Impressed by the elegance of Lehmann’s genetic approach, she applied and was accepted to her lab. In 1994, Amon arrived in the United States, not knowing that it would become her permanent home or that she would eventually become a professor.
While Amon’s love affair with fruitfly genetics would prove short, her promise was immediately apparent to Lehmann, now director of the Whitehead Institute. “I will never forget picking Angelika up from the airport when she was flying in from Vienna to join my lab. Despite the long trip, she was just so full of energy, ready to talk science,” says Lehmann. “She had read all the papers in the new field and cut through the results to hit equally on the main points.”
But as Amon frequently was fond of saying, “yeast will spoil you.” Lehmann explains that “because they grow so fast and there are so many tools, ‘your brain is the only limitation.’ I tried to convince her of the beauty and advantages of my slower-growing favorite organism. But in the end, yeast won and Angelika went on to establish a remarkable body of work, starting with her many contributions to how cells divide and more recently to discover a cellular aneuploidy program.”
In 1996, after Lehmann had left for New York University’s Skirball Institute, Amon was invited to become a Whitehead Fellow, a prestigious program that offers recent PhDs resources and mentorship to undertake their own investigations. Her work on the question of how yeast cells progress through the cell cycle and partition their chromosomes would be instrumental in establishing her as one of the world’s leading geneticists. While at Whitehead, her lab made key findings centered around the role of an enzyme called Cdc14 in prompting cells to exit mitosis, including that the enzyme is sequestered in a cellular compartment called the nucleolus and must be released before the cell can exit.
“I was one of those blessed to share with her a ‘eureka moment,’ as she would call it,” says Rosella Visintin, a postdoc in Amon’s lab at the time of the discovery and now an assistant professor at the European School of Molecular Medicine in Milan. “She had so many. Most of us are lucky to get just one, and I was one of the lucky ones. I’ll never forget her smile and scream — neither will the entire Whitehead Institute — when she saw for the first time Cdc14 localization: ‘You did it, you did it, you figured it out!’ Passion, excitement, joy — everything was in that scream.”
In 1999, Amon’s work as a Whitehead Fellow earned her a faculty position in the MIT Department of Biology and the MIT Center for Cancer Research, the predecessor to the Koch Institute. A full professor since 2007, she also became the Kathleen and Curtis Marble Professor in Cancer Research, associate director of the Paul F. Glenn Center for Biology of Aging Research at MIT, a member of the Ludwig Center for Molecular Oncology at MIT, and an investigator of the Howard Hughes Medical Institute.
Her pathbreaking research was recognized by several awards and honors, including the 2003 National Science Foundation Alan T. Waterman Award, the 2007 Paul Marks Prize for Cancer Research, the 2008 National Academy of Sciences (NAS) Award in Molecular Biology, and the 2013 Ernst Jung Prize for Medicine. In 2019, she won the Breakthrough Prize in Life Sciences and the Vilcek Prize in Biomedical Science, and was named to the Carnegie Corporation of New York’s annual list of Great Immigrants, Great Americans. This year, she was given the Human Frontier Science Program Nakasone Award. She was also a member of the NAS and the American Academy of Arts and Sciences.
Lighting the way forward
Amon’s perseverance, deep curiosity, and enthusiasm for discovery served her well in her roles as teacher, mentor, and colleague. She has worked with many labs across the world and developed a deep network of scientific collaboration and friendships. She was a sought-after speaker for seminars and the many conferences she attended. In over 20 years as a professor at MIT, she has mentored more than 80 postdocs, graduate students, and undergraduates, and received the School of Science’s undergraduate teaching prize.
“Angelika was an amazing, energetic, passionate, and creative scientist, an outstanding mentor to many, and an excellent teacher,” says Alan Grossman, the Praecis Professor of Biology and head of MIT’s Department of Biology. “Her impact and legacy will live on and be perpetuated by all those she touched.”
“Angelika existed in a league of her own,” explains Kristin Knouse, one of Amon’s former graduate students and a current Whitehead Fellow. “She had the energy and excitement of someone who picked up a pipette for the first time, but the brilliance and wisdom of someone who had been doing it for decades. Her infectious energy and brilliant mind were matched by a boundless heart and tenacious grit. She could glance at any data and immediately deliver a sharp insight that would never have crossed any other mind. Her positive attributes were infectious, and any interaction with her, no matter how transient, assuredly left you feeling better about yourself and your science.”
Taking great delight in helping young scientists find their own “eureka moments,” Amon was a fearless advocate for science and the rights of women and minorities and inspired others to fight as well. She was not afraid to speak out in support of the research and causes she believed strongly in. She was a role model for young female scientists and spent countless hours mentoring and guiding them in a male-dominated field. While she graciously accepted awards for women in science, including the Vanderbilt Prize and the Women in Cell Biology Senior Award, she questioned the value of prizes focused on women as women, rather than on their scientific contributions.
“Angelika Amon was an inspiring leader,” notes Lehmann, “not only by her trailblazing science but also by her fearlessness to call out sexism and other -isms in our community. Her captivating laugh and unwavering mentorship and guidance will be missed by students and faculty alike. MIT and the science community have lost an exemplary leader, mentor, friend, and mensch.”
Amon’s wide-ranging curiosity led her to consider new ideas beyond her own field. In recent years, she has developed a love for dinosaurs and fossils, and often mentioned that she would like to study terraforming, which she considered essential for a human success to life on other planets.
“It was always amazing to talk with Angelika about science, because her interests were so deep and so broad, her intellect so sharp, and her enthusiasm so infectious,” remembers Vivian Siegel, a lecturer in the Department of Biology and friend since Amon’s postdoctoral days. “Beyond her own work in the lab, she was fascinated by so many things, including dinosaurs — dreaming of taking her daughters on a dig — lichen, and even life on Mars.”
“Angelika was brilliant; she illuminated science and scientists,” says Frank Solomon, professor of biology and member of the Koch Institute. “And she was intense; she warmed the people around her, and expanded what it means to be a friend.”
Amon is survived by her husband Johannes Weis, and her daughters Theresa and Clara Weis, and her three siblings and their families.
The Alana Center Presents: The New England Down Syndrome Symposium

On November 10, 8:45 a.m. to 4 p.m., join us for a series of stimulating talks addressing some of the latest research in New England and beyond.
This event will be held online. Registration is free but required. Click here to register.
If you have registered, click here to view the symposium.

This event was co-organized by The Alana Down Syndrome Center, The Massachusetts Down Syndrome Congress and The Lumind IDSC Foundation
Schedule:
8:45 – 9:00am Introduction (featuring remarks from Kate Barlett, MDSC Self-Advocate Advisory Council)
Session 1: Developmental biology
9:00 – 9:40am Elizabeth Fisher (keynote), University College London : Working with mouse models to understand Down syndrome
9:40 – 10:05am Laurie Boyer, MIT, Getting to the Heart of Down Syndrome
10:05 -10:30am Tarik Haydar, Center for Neuroscience Research, Children’s National Hospital, Variation in phenotypic presentation of mouse models of Down syndrome, findings and implications
10:30 -10:55am Lindy Barrett, Broad Institute, MIT and Harvard, Probing molecular convergence between Down syndrome and Fragile X syndrome
10:55 – 11:10am Break
Session 2: Alzheimer’s Disease in Down Syndrome – Recent Advances
11:10 -11:35am Diana Rosas, Massachusetts General Hospital Neurology
11:35am – 12:00pm Stephanie Santoro Massachusetts General Hospital Down Syndrome Program, LIFE-DSR study and assessment scales for AD in DS
12:00 -12:25pm Li-Huei Tsai and Diane Chan, Alana Down Syndrome Center at MIT, Leveraging Brain Rhythms As A Therapeutic Intervention For Alzheimer’s Disease
12:25 – 12:45pm Tribute to Angelika Amon (featuring remarks from Li-Huei Tsai, Claudia Moreira, Manolis Kellis, Laurie Boyer, Brian Skotko, Ed Boyden, and Emily Niederst)
12:45 – 1:30pm Lunch
Session 3: Dosage compensation/aneuploidy
1:30 -2:10pm Jeannie Lee (keynote), Harvard Medical School, Manipulating X-chromosome dosage to treat human disorders
2:10 – 2:35pm Stefan Pinter , University of Connecticut, Trisomy 21 silencing: Towards a dynamic in vitro model of Down Syndrome
2:35 – 3:00pm Mitzi Kuroda, Harvard Medical School , X chromosome dosage compensation in Drosophila
3:00 – 3:15pm Break
Session 4: Sleep Apnea in Down Syndrome
3:15 – 3:40pm Daniel Combs, University of Arizona, Medications for Obstructive Sleep Apnea in Children with Down Syndrome
3:40 – 4:05pm Christopher Hartnick, Harvard Medical School, HGN Implant for Severe Sleep Apnea: Where are we now?
4:05 pm Closing Remarks and Angelika Amon Tribute Video