With early studies indicating that non-invasive sensory stimulation of a gamma frequency brain rhythm may improve cognition in Alzheimer’s disease, researchers in the Alana Down Syndrome Center and The Picower Institute for Learning and Memory at MIT are beginning to also test it with volunteers from the Down syndrome community.
At a presentation March 23 in Worcester, Mass., at the Massachusetts Down Syndrome Congress 40th Anniversary Conference, Alana Center and Picower Institute Director Li-Huei Tsai said her research team is recruiting volunteers to try out the potential therapy, which involves flickering light and clicking sound at 40Hz, a gamma band frequency of the brain associated with processing sensory information and also memory and cognition.
Finding a safe and accessible way to enhance cognition is one of the goals of the team of researchers at the Alana Center, founded in 2019 at MIT with a gift from the Alana Foundation, Tsai said.
“The mission is to conduct research and develop technologies that help give people with disabilities the skills to participate in education, the workforce and in other community settings,” she said.
Addressing Alzheimer’s
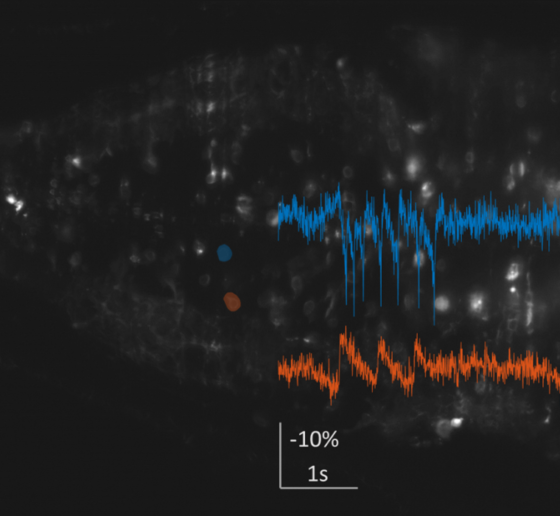
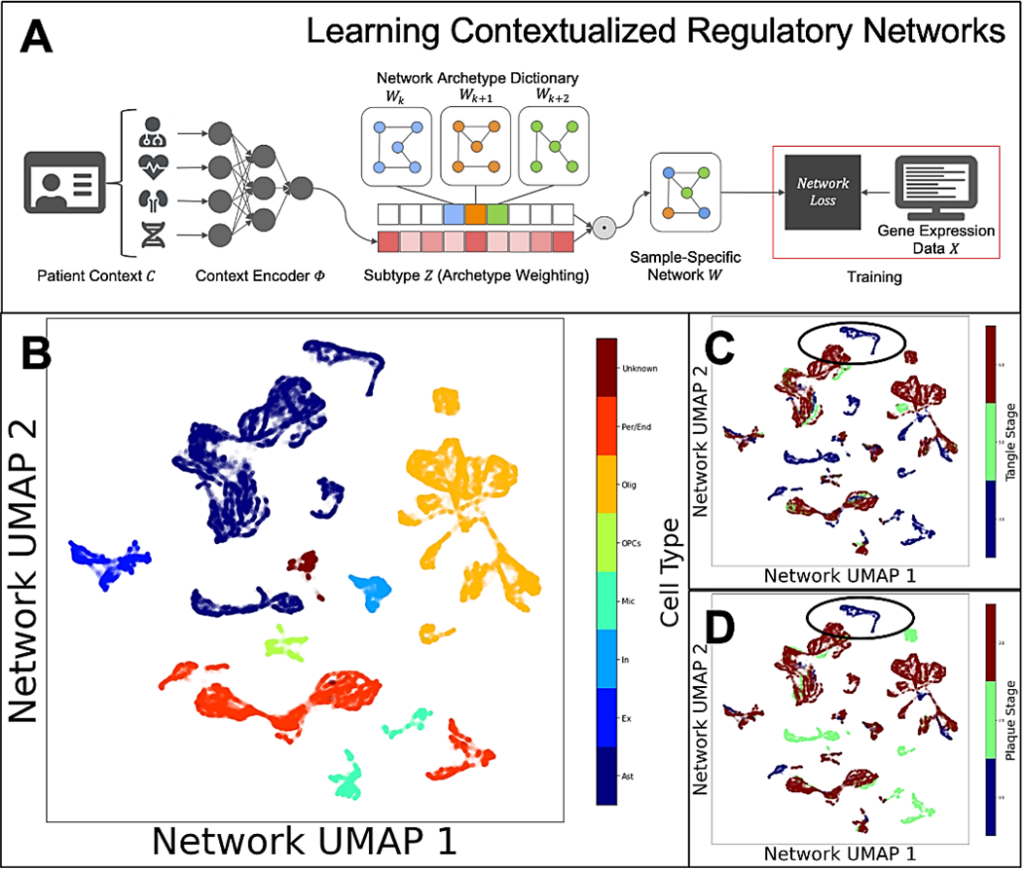
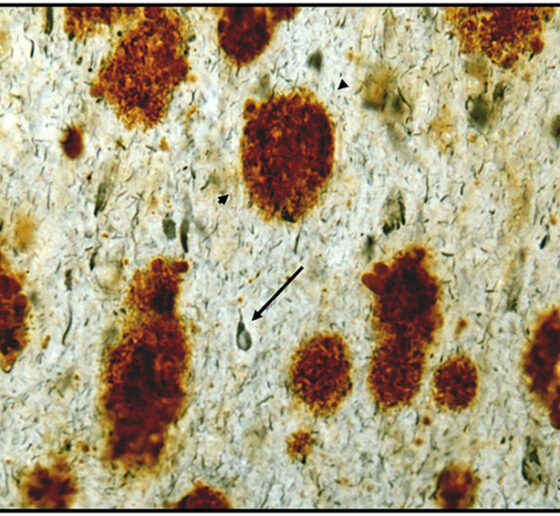
In several studies, Tsai’s team and others have tested forms of non-invasive 40Hz stimulation in mice modeling Alzheimer’s disease and with human volunteers with the condition. Mouse studies at MIT have shown significant reductions in disease pathology, including in levels of amyloid and tau proteins, and important health-promoting changes in brain cells including neurons, astrocytes and the brain’s vasculature. One of the lab’s most recent studies showed that 40Hz stimulation appears to clear amyloid by improving how well it can be flushed out by the brain fluids that flow through the glymphatic system. Importantly mice treated with 40Hz light, sound or vibration have also shown cognitive improvements.
In human volunteers, early-stage clinical studies at MIT and by a company that licenses MIT’s technology, have reported that the treatment is safe and produced some clinical improvements as well. Volunteers with Alzheimer’s disease exposed to 40Hz light and sound experienced benefits including a slower loss of brain volume and, in the company’s recently published phase 2 clinical trial results, also exhibited significantly slower declines on scores of mental functioning.
New Down syndrome study
Decades ago the life expectancy of people with Down syndrome was very low but today, many speakers at the MDSC conference noted, it extends past 60 years. But after the age of 40 the prevalence of Alzheimer’s disease among people with Down syndrome becomes very high. Many researchers believe that a major reason is that the amyloid precursor protein gene, a risk factor for Alzheimer’s, resides on chromosome 21, which is the chromosome that has an extra copy in Down syndrome.
Given the importance of Alzheimer’s disease and the overlap in disease pathology for people with Down syndrome, Tsai’s team at the Alana Center recently began to test whether 40Hz sensory stimulation could help.
As in the Alzheimer’s experiments, Tsai’s team started with mice. The data so far is preliminary—it has not yet been peer-reviewed and published—but Down syndrome model mice treated with 40Hz stimulation appear to show improved cognition.
The team has also begun a clinical study of people with Down syndrome. Testing so far is finding that light or light and sound combined increases the strength of the 40Hz gamma rhythm in the brain, as it does in Alzheimer’s patients and mouse models. With more volunteers, the team hopes to determine if the stimulation produces cognitive or functional benefits, Tsai said. The team is especially looking to include people in their 20s and 30s before the onset of Alzheimer’s disease symptoms.
Time, and testing, will tell if non-invasive gamma rhythm stimulation can benefit people with Down syndrome.