40Hz light and sound in sleep and neurogenesis
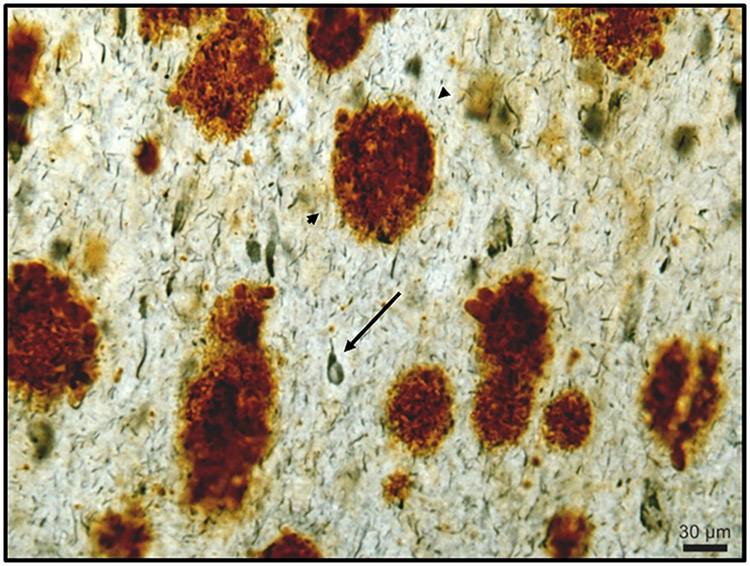
By the age of 40, most individuals with DS present a substantial accumulation of amyloid beta (Aβ) plaques and neurofibrillary tangles (NFT), toxic protein deposits considered to be hallmarks of Alzheimer’s disease. Pioneering studies performed by the Tsai laboratory have shown that sensory stimulation (i.e., light and sound) at the frequency of a key brain rhythm (gamma: 40Hz), a technique called GENUS, enhances gamma rhythms in the brain and can have neuroprotective effects in the context of Alzheimer’s disease. Recent work from the Tsai lab suggests this to be true in mouse models of DS as well. Recent work suggests a key mechanism through which GENUS exerts a neuroprotective effect is by promoting the clearance of waste products through the glymphatic system. The strongest known regulator of glymphatic clearance of waste is sleep. Therefore, a new research project aims to investigate whether GENUS can be performed during sleep and to assess how the potential neuroprotective effects of this treatment compare to the effects of using GENUS during wakefulness. The results of this project could provide a pathway to enhance the beneficial effects of GENUS in the context of Alzheimer’s disease and DS. Moreover, the ability to deliver GENUS during sleep in future clinical trials or interventions would lead to a striking increase in compliance with the treatment and the daily amount of stimulation, particularly for individuals with DS. Taken together, the administration of GENUS during sleep promises a striking increase in treatment efficacy.
 To address this aim, Alana Fellow Cristina Blanco-Duque has built a setup that supports continuous (24h/7 days a week) electrophysiology recordings in mice and is equipped to perform GENUS. She also established multi-site in vivo electrophysiology recordings in a mouse model for DS. This setup has already produced several rich datasets with exciting results, including that the DS model mice show fragmented and lighter sleep than their wild-type littermates, and may have hyper-synchronized brain rhythms.
To address this aim, Alana Fellow Cristina Blanco-Duque has built a setup that supports continuous (24h/7 days a week) electrophysiology recordings in mice and is equipped to perform GENUS. She also established multi-site in vivo electrophysiology recordings in a mouse model for DS. This setup has already produced several rich datasets with exciting results, including that the DS model mice show fragmented and lighter sleep than their wild-type littermates, and may have hyper-synchronized brain rhythms.
Blanco-Duque and Tsai lab colleagues have also developed a stimulation system that allows the lab to deliver GENUS selectively during sleep or wake. This setup will be used to test whether delivery of the GENUS light and sound treatment is possible during sleep, and how this affects sleep/wake patterns. This system can also be used to study the effects of GENUS on the clearance of toxic waste products during sleep.
The lab has also found in early results that after three weeks of 40 Hz light and sound (GENUS) stimulation in the mouse model of DS there is a striking benefit of the production of new neurons in the adult brain. This process has been shown previously to be significantly impaired in mouse models of DS, which may contribute to some of the cognitive dysfunction that has been observed. The lab is also investigating the effects of extended GENUS treatment on several behavioral tasks, including spatial short-term memory and anxiety behaviors, as well as markers of inflammation and immune activity in the brain.
Based on preliminary data using single-nucleus RNAseq – a high-resolution method of quantifying gene expression in individual brain cells – the lab hypothesizes that GENUS may alter the expression of several critical DS-related genes that influence the balance between the production of new astrocytes and neurons.
GENUS human clinical studies

Work has started within the Tsai lab to translate the findings in mice into benefits for human subjects with DS. Clinical work began by investigating the safety, compliance, and entrainment of the 40 Hz stimulus. In a recent publication, the Tsai lab found that repeating auditory tones can also be used to entrain gamma oscillations, and in combination with visual stimulation, GENUS can impact brain regions outside of the primary sensory cortex and extend into the hippocampal memory. With combined visual and auditory stimulation at 40Hz, the entrainment of gamma oscillations can be seen across the auditory cortex, visual cortex, hippocampus, and medial prefrontal cortex. Based on these studies, the team developed a non-invasive medical device that will possibly be effective in preventing the progression of AD and other pathology in individuals with DS. Our objective is to determine whether non-invasive sensory stimulation can be used to modulate gamma power and synchronization in individuals with DS as a potential therapeutic to prevent AD and other cognitive troubles in this population.
In a first Phase 1/2 study, the team will treat 30 individuals with DS and age-matched, cognitively typical controls with the GENUS light and sound device while using electroencephalography (EEG) to evaluate induced entrainment and effects on their brain circuitry. Participants are blindly randomized to receive 1-hour of either sham or active 40Hz light and sound stimulation. The team performed cognitive testing before and after the stimulation session. Data so far suggests that our prototype GENUS light and sound device is safe and tolerable in participants with Down syndrome with no significant adverse effects and no evidence of seizure-like activity on EEG during treatment. The lab is continuing to recruit study subjects , with approximately 18 adults with DS already participating in the study.
Prenatal GENUS
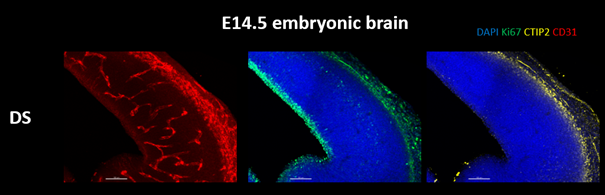
New work in the Tsai lab is finding that the effects of GENUS extend beyond the brain. GENUS stimulation in aged mice changes the number of intestinal immune macrophages and the microbiome composition of the gut. It has been previously shown that immune changes in pregnancy can have large effects on the fetus. Therefore, we started a new project in the lab to understand if GENUS treatment during pregnancy can improve deficiencies found in the brain development of Ts65Dn embryos. The lab is investigating changes in the reduction in brain size and cortical plate thickness observed in Ts65Dn embryos after maternal GENUS treatment. The lab will analyze the maternal plasma, placenta, and brain to understand the possible mechanisms by which GENUS ameliorates the embryonic brain. They also aim to understand if prenatal GENUS treatment can benefit the offspring postnatally in terms of their brain development and function.